
Humans and Technology / Brain-Computer Interface
A Shocking Way to Fix the Brain
Neurosurgeons hope to treat some of the most intractable mental disorders by putting advanced arrays of electrodes into patients’ brains.
When Emad Eskandar talks about one of his neurosurgery patients with obsessive compulsive disorder, he’s not talking about someone who arranges his record collection by color, size, and name. Or someone who ritualistically touches the knob on the stove twice before leaving the house and says, “Sorry, I’m a little OCD.”
Eskandar’s OCD patients take three-hour showers. They spend eight hours cleaning their surroundings with bleach. They get stuck at the bathroom sink in their hotel room on appointment days, unable to stop washing their hands until someone comes to get them. OCD affects an estimated 2.5 million adult Americans. But only those who have exhausted all other treatment options—Luvox, Anafranil, Prozac, cognitive behavioral therapy—end up on Eskandar’s operating table at Massachusetts General Hospital. By then, they are desperate enough to try almost anything—even deep brain stimulation (DBS), an option of last resort that Eskandar has spent the last 15 years mastering and refining.
In an initial surgery, Eskandar drills two dime-size holes in the top of the patient’s skull and sinks 42-centimeter-long electrodes about seven centimeters deep into the gray matter of the brain. In a second surgery, usually a couple of days later, he creates a pocket under the skin in the chest or abdomen, implants a device incorporating a battery and pulse generator into this newly created space, and runs a wire up to the skull, connecting it with the electrodes. When turned on, the device emits an electrical current that stimulates the neural fibers carrying information from primitive brain areas associated with motivation to the frontal lobe. In 50 percent of Eskandar’s cases, a miracle follows: the obsessions and compulsions fade and then disappear.
Though the treatment sounds extreme, in some respects his OCD patients are the lucky ones. There is no such FDA-approved last-resort option for the millions of Americans suffering from other psychiatric illnesses, such as depression, post-traumatic stress disorder, or schizophrenia. Or for borderline personality disorder and traumatic brain injuries. But for all these conditions, that may soon change.
Deep brain stimulation has been used for almost two decades to treat patients with severe forms of Parkinson’s (and since 2009 to treat a far smaller number of patients with OCD). As many as 125,000 people are living with electrodes implanted in their brains. As part of President Obama’s Brain Initiative, Eskandar is co-leading a team of doctors, scientists, and engineers that is one year into a five-year, $30 million effort to use DBS to treat severe psychiatric disorders, most of which have been considered too complex and mysterious for any such system currently on the market. Conditions like schizophrenia, PTSD, and depression are characterized by unpredictable changes in the brain that lead to intermittent episodes. Taming them will require a new kind of device capable not just of stimulating the brain but of monitoring brain activity in real time and detecting anomalies that, in many cases, neuroscientists have not yet identified.
It will be up to Eskandar, and the team he is leading with his longtime MGH collaborator Darin Dougherty, to identify how the brains of people suffering from these disorders differ from those of healthy individuals. And then they must figure out what kind of electrical stimulation patterns might be used to fix them. “We’re aiming for something ridiculously ambitious,” he acknowledges.
Engineers across the Charles River at Draper Laboratory are working closely with Eskandar to develop the needed hardware. They have built a prototype of a DBS system that will be able to record signals from hundreds of sites deep in the brain and on its surface. The device will use pattern recognition software to detect anomalous activity associated with pathological mental states; then it will stimulate areas of the brain in response. The Draper engineers are in the process of fabricating a miniaturized version of the device, which they hope to test out in humans as early as 2016.
Most psychiatrists agree that new treatments for mental illness are desperately needed. Existing drugs for brain disorders are often ineffective and frequently produce troublesome side effects. One reason is that drugs alter the chemistry of the entire brain, not just the area of interest, modulating the behavior of otherwise healthy neurons (see “Shining Light on Madness,” July/August 2014).
With electrical stimulation, on the other hand, doctors can target discrete populations of neurons, confining the treatment to small, isolated areas of the brain that are causing the problems. “DBS allows us to go into the actual circuit that we know is involved in a condition, and we’re stimulating it and making it fire or not fire in the way that we want it to,” says Dougherty, the psychiatrist teamed with Eskandar to direct Mass. General’s Division of Neurotherapeutics, the nation’s busiest center for psychiatric surgical treatment. “It’s night and day in terms of the robustness.”
To treat brain conditions this way, of course, the surgeons need to identify and understand the precise circuits that cause them—which in many cases has not yet been done. Though neuroscientists have learned a lot about how brain circuits are organized and how they function, it’s rarely been possible to watch these circuits operate in real time. But Eskandar and Dougherty say the technology they are designing and testing will open up that possibility. Recording multiple patches of neurons simultaneously for extended periods of time, they believe, will allow them to transform the way we define and understand different types of mental illness—and, more important, finally lead to more effective ways to treat them.
Calming the waters
Physicians have been experimenting with electricity to treat brain disorders since antiquity, in some cases even applying electric ray fish to the skull. But DBS was born in a French operating room in 1987, when a neurosurgeon named Alim Louis Benabid made a fortuitous discovery while preparing to operate on a patient suffering from uncontrollable trembling.
For decades, the last-gasp technique for such patients had been extreme but often quite effective: brain surgeons simply drilled holes into the skull and removed the areas of the brain thought to be causing the problem. The approach was sometimes used for other movement disorders, as well as severe epilepsy and some mental illnesses. That day in 1987, Benabid planned to remove a piece of his patient’s thalamus, a walnut-shaped structure deep in the brain. By destroying or “lesioning” part of the tissue, he intended to cut out the source of the stray electrical impulses flying down the peripheral nerve fibers of the body and causing his patient’s hand to shake.
Brain surgery of any sort, of course, is a high-stakes proposition. Miscalculations can cause paralysis, blindness, even death. To avoid surprises, Benabid took a common surgical precaution: he kept his patient awake in the operating room, which is possible because there are no neural pain receptors. He inserted an electrical probe into the part of the brain he intended to remove. Then he delivered a pulse and watched the patient closely to make sure the stimulation had no unanticipated effects. It’s a technique neurosurgeons have been using for well over half a century to verify that the area they are about to remove does not serve an essential function; the small current in the electrode causes the neurons around it to fire, revealing what, if any, role they play in bodily processes.
By 1987, neuroscientists had developed a protocol that Benabid fortunately decided to ignore. Instead of stimulating the brain of his patient at a frequency of 50 hertz, he turned the knob up to almost 100 hertz. When he applied the electrode to his target, something unexpected occurred: the patient’s hand stopped shaking—for the first time in years. When Benabid turned off the current, the shaking resumed. When he turned it back on, it stopped again. Stimulating at high frequency, he realized, somehow quieted the troublesome signals.
In 1991 he published a paper detailing his use of DBS to treat tremors on both sides of the body. He followed up with another landmark paper demonstrating that he could alleviate many of the other debilitating symptoms of Parkinson’s, including slowed movement and muscle rigidity. The U.S. Food and Drug Administration approved DBS for use in tremors in 1997 and for Parkinson’s in 2002. It has now been used on tens of thousands of patients.
Even so, years later, scientists are still debating why DBS works. Scientists had long known that uncontrollable trembling was somehow caused when errant signals emanating from structures deep in the brain continuously activated areas of the motor cortex controlling the body’s movements. By the 1980s, they even knew what was causing these signals in Parkinson’s—insufficient quantities of a chemical signaling agent called dopamine in structures called the basal ganglia. For decades, however, the organization of the basal ganglia and other features of the brain’s inner layers remained largely a matter of speculation.
Benabid theorized that stimulating the neurons suppressed the abnormal activity. Over the last decade or so in animal studies, neuroscientists have more precisely measured neuronal output and found that DBS seems, on the contrary, to stimulate activity. Philip Starr, a neurosurgeon at the University of California, San Francisco, who specializes in movement disorders, has articulated a leading theory: he believes that DBS works by “desynchronizing” firing patterns within circuits.
Just like energy moving through the ocean, electrical signals passing through the brain travel in waves. And as in an ocean storm, a big wave moving at the right speed can subsume all the little waves in its path. In Parkinson’s, abnormal activity builds on itself, creating pathological waves of activity that gain control of the circuit, drowning out all other activity. DBS breaks these waves up again, allowing the circuit to unlock and smaller signals to get through.
Whatever the mechanism behind DBS, it was only a matter of time before researchers began to consider how they might extend the technique to treat other brain disorders—in particular, intractable mental illnesses.
Depression
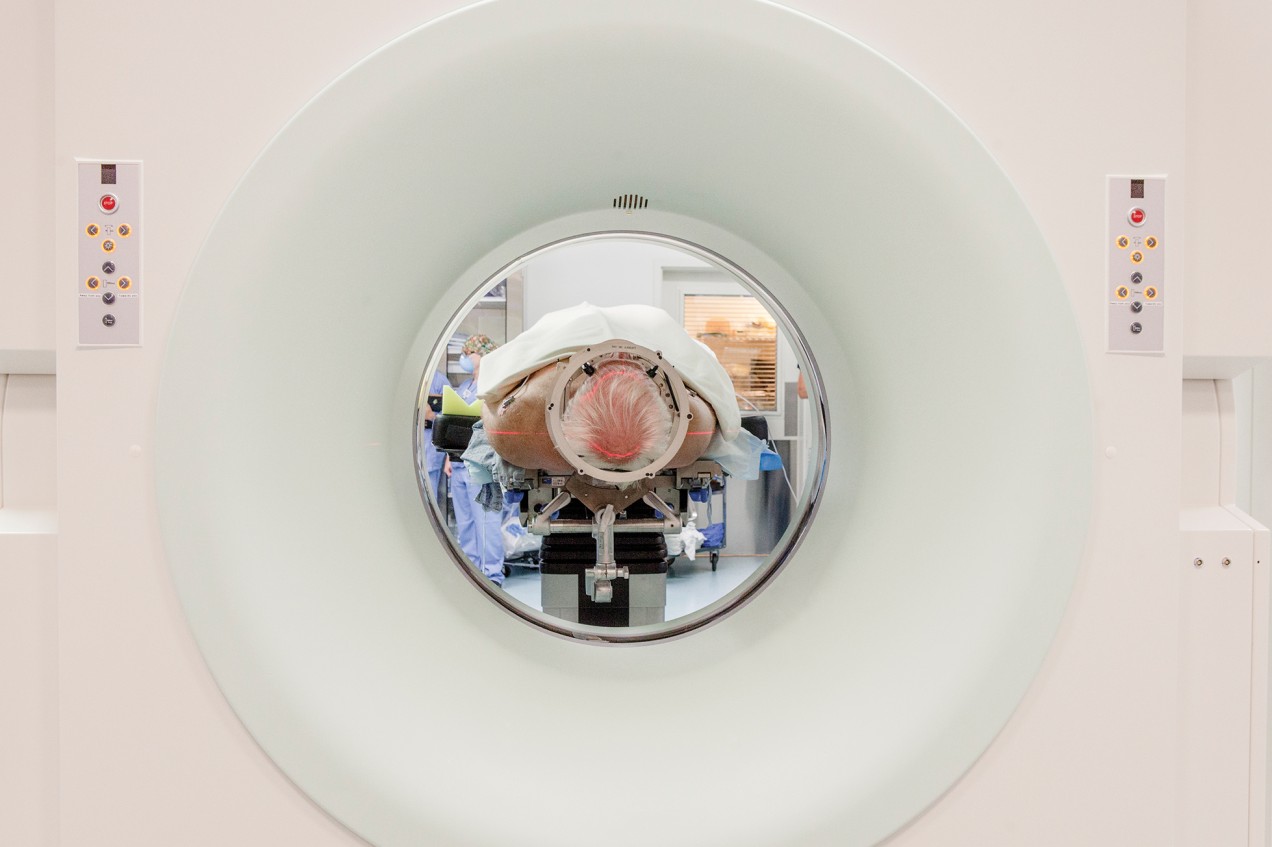
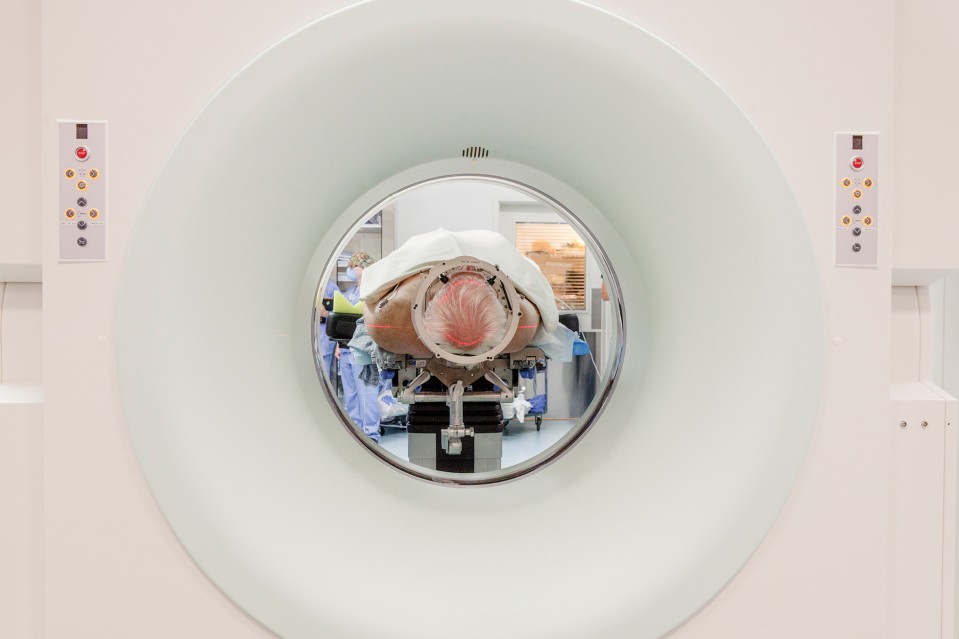
On the day I visit Emad Eskandar’s operating room at Mass. General, his patient is laid out on a stretcher wearing a festive dark-blue shade of fingernail polish. The patient is tormented by OCD and has failed to respond to all other treatment options. Now she lies anesthetized amid trays of shiny metallic scalpels and scissors, and the nurses have draped her in a white sheet. They have also shaved her head and, using clamps and screws, secured a sturdy, box-like frame to her forehead and the sides of her skull. Each arm of the frame is etched with the tiny numbers of a ruler, down to the millimeter. The numbers will allow Eskandar to precisely line up the hollow metal leads he plans to press through his patient’s cortex and into the center of her brain, following a straight route to his target.
First, however, the neurosurgeon needs to map that route. Eskandar sits nearby in scrubs, a surgical mask rakishly pushed onto his blue surgical cap, and moves a mouse pointer over a spot at the center of one of four images displayed on a monitor, depicting the patient’s brain. Each image is taken from a different angle. “This is perfect—you want to be here,” he tells a junior surgeon. “That’s your entry point.”
Eskandar has implanted electrodes in scores of OCD patients; he was among the first neurosurgeons to begin performing the intervention experimentally, long before it was approved for widespread use by the FDA in 2009. It was exactly the type of opportunity he had been hoping for when he decided to attend medical school.
He had excelled at math and physics in high school and entered the University of Nebraska intent on becoming a chemical engineer. But that changed when he got a night job working in a psychiatric institution, supervising patients experiencing acute psychotic breaks. The patients he met made a profound impression. There was the mathematics professor with a PhD from Northwestern, hopelessly confused by his own delusions. Eskandar also recalls a disheveled guy his own age, who heard voices in Van Halen songs and once hopped the fence during an outdoor recreation period when Eskandar wasn’t paying attention. The police found the patient a couple of hours later, standing in the middle of the freeway directing traffic with a fork.
Eskandar was fascinated by the magnitude of these delusions and amazed by how little doctors understood about mental illness. “It was a very different feel than a regular hospital,” he recalls. “It was like, ‘Does anybody really know what’s going on?’” He applied to medical school hoping to unlock the mysteries of the brain. After a stint doing brain research at the National Institutes of Health, he earned a residency at Mass. General Hospital just as the FDA was approving the first use of deep brain stimulation for movement disorders. Having entertained and kept watch over patients with brain disorders just a few years earlier, he now found himself operating on them, and in the process he got the opportunity to measure their neural activity and join the hunt for the causes of such bizarre behavior. He stayed at Mass. General after his residency ended.
Now, Eskandar is standing over the OCD patient’s naked scalp, marking his entry points with a Sharpie. Then he snaps an attachment onto the metal frame encasing the patient’s head, adjusts the angle to line up the numbers, and lets the assemblage of nurses, residents, and other observers know he is ready. Within a few minutes, he has opened up two boreholes in the patient’s skull and used the head rig to pilot two long, hollow metal tubes down through the outer layers of her brain and into the middle of the gray matter. Into the tubes he slides a pair of thin electrodes that will be connected to the device he plans to implant later. Then he removes the tubes, stitches the electrode leads into the scalp using silk threads, and fills the boreholes with fast-setting cement.
The device to be implanted in the patient’s body is effective at treating OCD. But it uses technology that has been around for decades, and the veteran neurosurgeon is certain that he and other clinicians have only scratched the surface of what might be possible once today’s technologies upgrade the DBS systems available in the operating room. “Think about what’s happened over the past 20 years in terms of miniaturization and Moore’s Law and everything,” Eskandar says. “You have this device that came out in the ’90s. When it was designed in the 1980s, I didn’t even have a cell phone.”
The new system being developed at Draper, which Eskandar and his Mass. General colleagues helped design, will be able to gather data from as many as 320 electrodes—including multiple groups of sensors placed on the outer layer of the brain—and deliver stimulation accordingly. Instead of a bulky processor implanted in a patient’s chest or abdomen, the device will consist of a miniaturized central hub, smaller than a cell phone, with an integrated battery. The whole thing will be compact enough to fit snugly on the back of the skull. The skull hub will attach to as many as five ceramic and titanium electronic satellites that are small enough to fit into dime-size burr holes drilled into the top of the skull. Each of these satellites will collect and relay the data from the electrodes that will be connected to the sensors or the leads deep in the brain. The team has also created a remote base station that communicates wirelessly with the skull hub; it can recharge the hub’s battery and analyze the data it has stored over the course of the day.
The new device, with its multiple leads and sensors, could be key if Eskandar and his colleagues are to extend the technology to depression and other, more complicated mental disorders. In the mid-2000s, he and Dougherty won approval to conduct a trial that used DBS to treat depression. The results, in some cases, were remarkable, hinting at the potential the team is now attempting to realize. But in many other cases, the treatment was frustratingly ineffective. A more advanced device could mean far more precise interventions tailored to individual patients and, perhaps, an effective treatment for a larger group of people.
Their first patient had tried all the medications that science had to offer, not to mention 30 rounds of electroconvulsive therapy. Her name was Liss Murphy, and by the time she met Dougherty in 2006,she was desperate. A couple of years earlier, she had been a dynamic, 30–something PR executive living in Chicago. But depression had incapacitated her in a matter of weeks, leaving her hardly able to speak. One day she left work and never went back. Forced to move home to the Boston area in 2004, she ended up at McLean Hospital.
After Eskandar operated on Murphy, she began an astonishing recovery. She was able to resume her relationships with friends and family. She had a son, and experienced happiness, laughter, and joy again for the first time in years. The power of the approach was driven home to her in 2012, when an infection required doctors to shut off Murphy’s device for several months. Within days, her depression returned; but when the device was turned back on, she says, she experienced a powerful physical transformation.
“It was just a surge of warmth that rises through you, and I could tell it was on,” she says. “I woke up the day after and it was a whole new world. The colors outside were brighter. My son and I went to the story hour. It had been months since just he and I had done anything. Everything was new again, and it was like I made it to the other side.”
Inspired, Dougherty and Eskandar expanded their trials and saw similar results with a number of other patients (though certainly not all). By then, a parallel effort to use DBS against depression was already under way. In March 2003, Helen Mayberg, a neurologist then at the University of Toronto, had implanted a DBS device into a patient with depression, placing it in a narrow band of a brain structure called the subgenual cingulate. She published a paper in the journal Neuron in 2005, a year before Murphy’s operation, reporting results in six subjects (she followed that up with a group of 20, who are still being followed today). Like Murphy, some of them had been virtually catatonic before the surgery but recovered.
Mayberg’s initial success with DBS, along with the work of Eskandar and Dougherty’s group, fed the expectation that the device would soon win FDA approval for a condition affecting millions of Americans. Both groups had somewhere around a 50 percent response rate, with remission in a third of the cases, according to Dougherty. But the large trials the FDA mandated before the treatment could be approved required control groups to measure placebo effects. Experimenters implanted DBS devices in all the volunteers; then they randomly assigned half to a standard protocol of stimulation and the other half to a protocol in which the electrode is never turned on. After analyzing preliminary results, the FDA halted both trials. “We ended up having a fairly high placebo effect,” Dougherty says. “But it definitely worked in some people.”
Eskandar and Dougherty have seen too many remarkable recoveries to discount the treatment. Mayberg also remains a staunch believer in the power of DBS to treat depression. All three, however, believe that a more sophisticated DBS system of the sort Draper is developing is likely to make the therapy more effective. The reason is simple: the problems that occur in depression and other psychological disorders are not confined to one anatomical location. They are diseases of neural circuits and usually present complex arrays of symptoms, which might vary depending on which part or parts of the circuit are affected. This means there are different varieties of depression, and different varieties of patients with depression; each person might respond differently depending on where, when, and how the brain is stimulated.
In recent years, Mayberg has begun to map the complex connections radiating out from the spot she targets in DBS, a region called area 25. Working backwards, she hopes to reverse-engineer the circuit and identify all its hubs and component parts. With a more complex device capable of sensing and stimulating in multiple areas, she believes, it might be more feasible to tailor interventions to different subjects, adapting stimulation patterns to their specific symptoms and neural activation patterns.
Eskandar and Dougherty, meanwhile, have even broader ambitions. They hope to develop therapies for a whole host of other mental conditions so complex that treating them with the current generation of crude, one-directional devices would be virtually unimaginable.
Telltale colors
Sitting in Eskandar’s lab, I watch a rotating 3-D image of a translucent skull and the brain within it. Within the black-and-white brain, distinct neural activation patterns are highlighted in three different colors: turquoise, orange, and magenta. To create the images, Eskandar’s colleagues used functional magnetic resonance imaging (fMRI), a technique that tracks changes in neural activity by measuring blood flow to different areas of the brain. The turquoise represents the brain activation patterns recorded from a healthy subject as he performed a specific task. The orange and magenta represent the activation patterns recorded from the brains of two psychiatric patients as they performed the same task. All three patterns appear different. Although the orange and magenta patients have both been diagnosed with major depression, each has an additional condition: one is suffering from PTSD and the other has generalized anxiety disorder.
“These disorders, by very definition, are constellations of symptoms,” Dougherty says. Which is why, he argues, a more precise treatment, better tailored for individual patients, could make all the difference. “There is no depression spot,” he says. “There is no PTSD spot. There is no borderline personality disorder spot.”
Using the DBS system that’s currently available, Eskandar explains as he points at the two depressed patients’ brain patterns, the treatment strategy would be simply to turn on an electrode and stimulate the same area of the brain for both patients. The advanced DBS system Dougherty and Eskandar are developing with Draper, in contrast, will be able to sense abnormal patterns of brain activity in real time and stimulate whichever areas are affected. They should adjust when new patterns crop up, applying a jolt of electricity in the right spot each time.
Eskandar once again calls my attention to the screen. The three brain scans we are looking at, he tells me, were all recorded while the patients performed a task that measured their ability to quiet the emotional areas of the brain and answer a question that required focus and mental clarity. Eskandar points to one of the depressed patients’ brain activation patterns, explaining that it is the same pattern one usually finds in patients experiencing symptoms of PTSD. The emotion-driven part of the brain called the amygdala is alight with activity. It is firing far more robustly than the amygdalas of normal patients performing this same task. It’s as if the emotional part of this patient’s brain is screaming, drowning everything else out.
Imagine, Eskandar suggests, if we could simply override this reaction, manually activating and deactivating the appropriate areas. In fact, he has already attempted to demonstrate just that in a patient who had electrodes implanted in preparation for surgery to treat epilepsy (neurosurgeons often use this technique to monitor activity and verify the precise location from which seizures are originating). Eskandar and his team could turn up the patient’s emotional response to a picture of a human face by stimulating the amygdala, and they were able to blunt that response by stimulating a different area, the dorsal anterior cingulate cortex.
The team hopes to design a whole host of new DBS treatments: the device’s electrodes will be inserted at locations chosen according to each person’s constellation of symptoms, and the particular abnormalities in the brain circuits will determine where the current will be activated. Eskandar is optimistic about the prospects for treating depression with these new tools. He also has high hopes for treating PTSD and generalized anxiety disorder. He even feels good about the possibilities for treating addiction, schizophrenia, and traumatic brain injury. But he acknowledges that some of the conditions he and Dougherty plan to target, such as borderline personality disorder, remain long shots. Even in the one psychiatric disorder for which DBS is FDA-approved, OCD, the success rate still hovers around 50 percent—a stark reminder of the challenges that lie ahead.
Indeed, Eskandar and Dougherty are under no illusions. The human brain remains one of the most enigmatic and complex biological systems known. And in many ways our efforts to understand it are still in their infancy. By the end of this year, Eskandar says, he hopes to demonstrate that the new system can be programmed to sense a specific pattern of brain activity and respond to it. It’s a relatively simple test of the technology. Even so, success is not assured. “I’m sure it’s not going to work the first time, or probably even the third time,” he says. “But eventually it’s going to work. And we’ll keep trying until we get it right.”
Adam Piore is a freelance journalist based in New York.
Advertisement