
Capturing Wasted Methane
New process from MIT could turn waste greenhouse gas into a useful resource.
Methane gas, a vast natural resource, is often burned off at oil wells in a process called flaring, which currently wastes 150 billion cubic meters of the gas each year and generates a staggering 400 million tons of carbon dioxide. That contributes significantly to global warming. Letting the gas escape unburned would lead to even greater environmental harm, however, because methane is a much more potent greenhouse gas than carbon dioxide is.
Why is all this methane being wasted? The answer, as the saying goes in the real estate business, is simple: location, location, location.
In places where it is convenient to do so, methane that would otherwise be flared off when drilling for petroleum is captured and used to generate electrical power or produce chemicals. However, special equipment is needed to cool and pressurize methane gas, and pressurized containers or pipelines are needed to transport it. In many places, such as offshore oil platforms or remote oil fields far from the needed infrastructure, that’s just not economically viable.
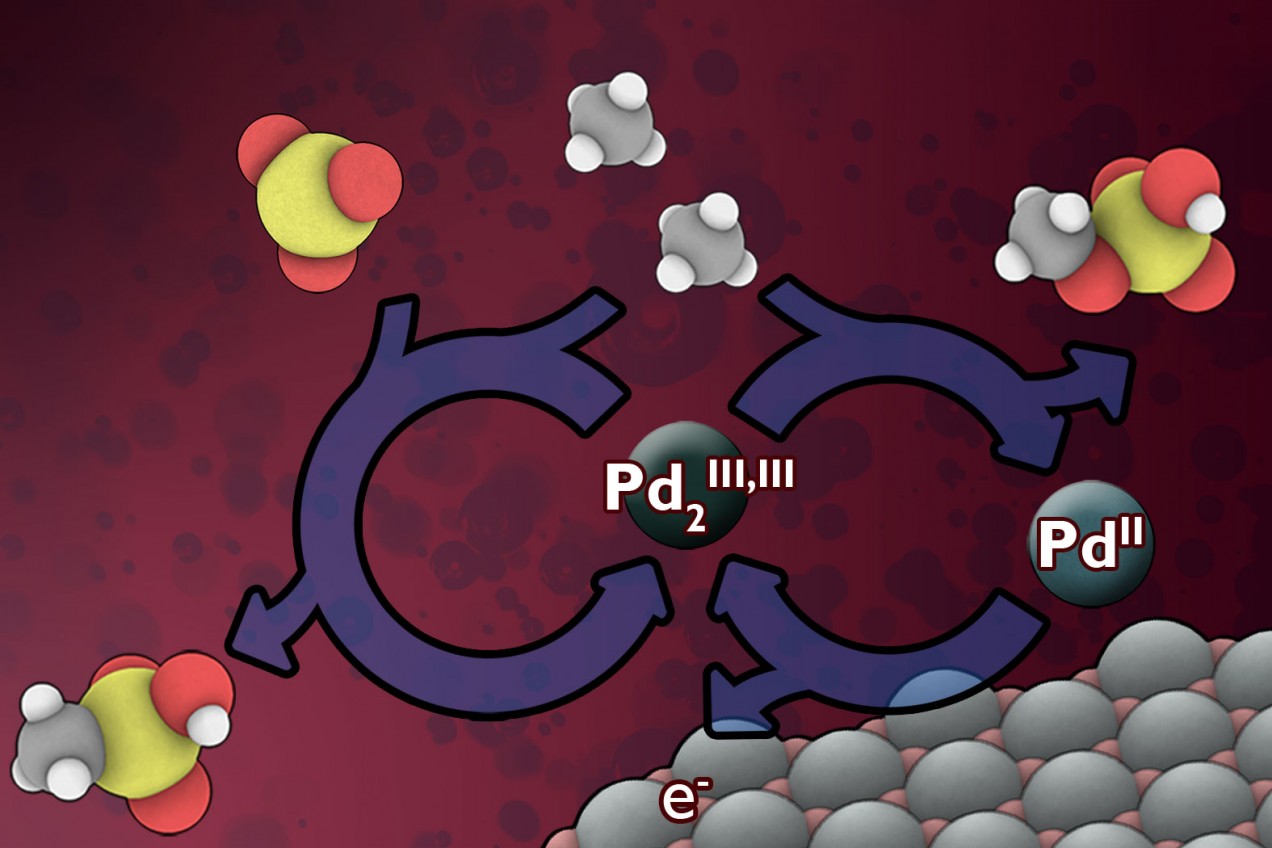
But now, assistant professor of chemistry Yogesh Surendranath, PhD ’11, and three colleagues have found a way to use electricity, which could potentially come from renewable sources, to convert methane into derivatives of methanol, a liquid that can be made into automotive fuel or used to produce a variety of chemical products. This new method, using electrically generated catalysts, may allow for lower-cost methane conversion at remote sites, and it could pave the way to making use of a significant methane supply that is otherwise totally wasted.