
Good news for joints
A new drug delivery material could help prevent cartilage breakdown.
Osteoarthritis, a disease that causes severe joint pain, affects more than 20 million people in the United States. Some drug treatments can help alleviate the pain, but there are no treatments that can reverse or slow the cartilage breakdown associated with the disease.
In an advance that could improve the treatment options available for osteoarthritis, MIT engineers have designed nanoparticles that can administer drugs directly to the cartilage. The material can penetrate deep, delivering drugs that could potentially heal damaged tissue.
“This is a way to get directly to the cells that are experiencing the damage and introduce different kinds of therapeutics that might change their behavior,” says Paula Hammond ’84, PhD ’93, head of MIT’s Department of Chemical Engineering, a member of MIT’s Koch Institute for Integrative Cancer Research, and the senior author of the study.
In rats, Hammond and her colleagues showed that delivering an experimental drug called insulin-like growth factor 1 (IGF-1) in this manner enabled cartilage regeneration much more effectively than injecting the drug into the joint on its own. The paper detailing the results of their study appeared in the journal Science Translational Medicine.
The researchers designed their material so that it would remain in the joint after being injected—unlike existing arthritis drugs, which are often cleared from the joint before they can reach the deep layer of chondrocytes that they are meant to target.
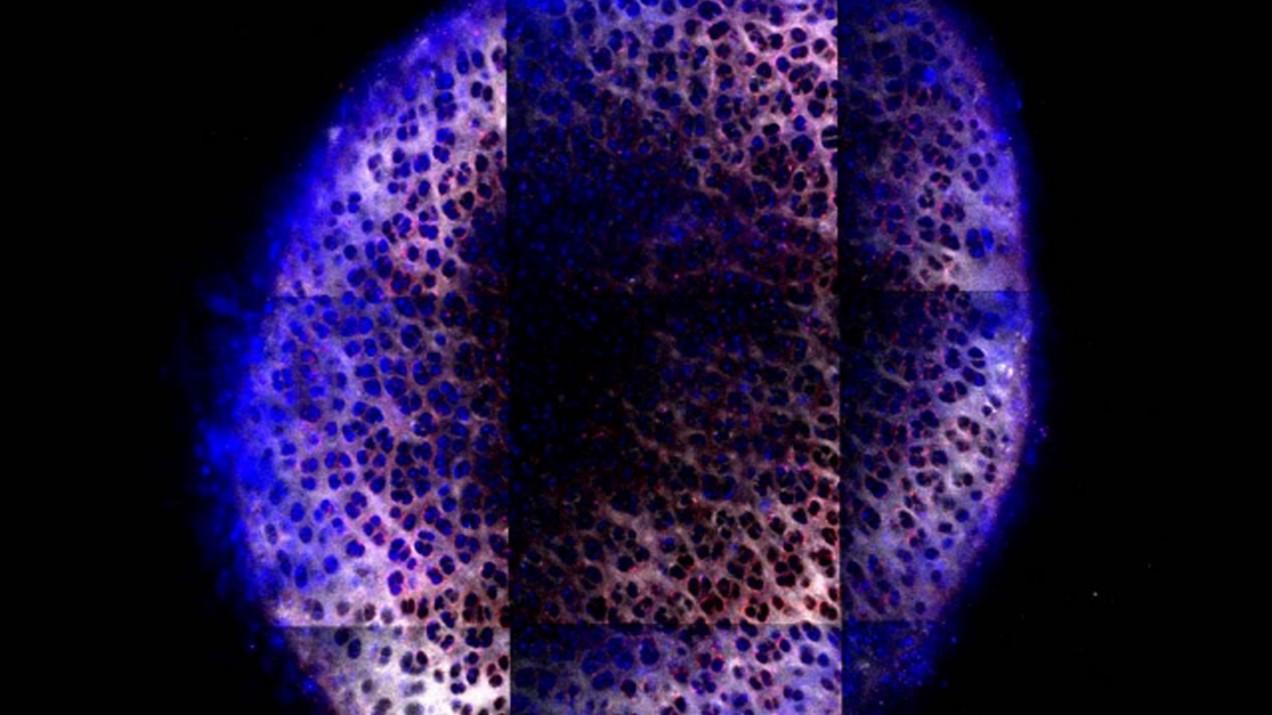
The spherical particles they developed contain many structures called dendrimers that have branches diverging from a central core. There is a positive charge at the tip of each branch, which helps the particle bind to the negatively charged cartilage, and molecules of IGF-1 are also attached to the surface. This design helps the particles to coat the surface of cartilage and then diffuse through it.
Once the particles reach the chondrocytes, the IGF-1 molecules bind to receptors on the cell surfaces and stimulate the cells to start producing proteoglycans, the building blocks of cartilage and other connective tissues. The IGF-1 also promotes cell growth and prevents cell death.
When the researchers injected the particles into the knee joints of rats, they found that the material had a half-life of about four days, which is 10 times longer than IGF-1 injected on its own. The drug concentration in the joints remained high enough to have a therapeutic effect for about 30 days.
The researchers began developing this material as a way to treat osteoarthritis that arises after traumatic injury, but they believe it could also be adapted to treat age-related osteoarthritis. They now plan to explore the possibility of delivering different types of drugs, such as other growth factors, drugs that block inflammatory cytokines, and nucleic acids such as DNA and RNA.