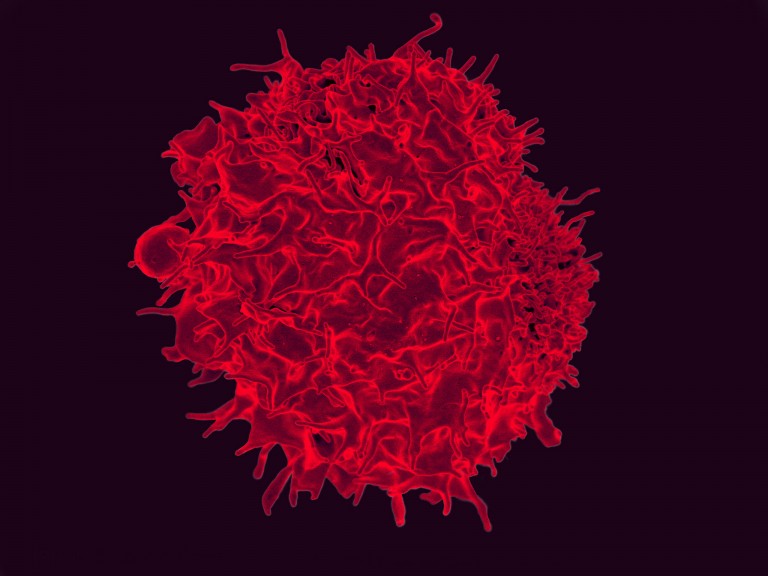
Progress in an area of cancer treatment known as CAR-T therapy has been swift—but it's a life-and-death game. Never has this been more clear than in the past few days.
Late on Monday, the French biotech firm Cellectis announced that its clinical trials involving genetically modified immune cells to treat two different types of cancer had been halted by the FDA after a patient was killed (Cellectis's stock took a beating on the announcement). The trials aimed to prove that a more advanced version of CAR-T, known as "off the shelf" or "universal" CAR-T, could work. The treatment tweaks T cells from a donor to turn them into cancer-killers and then infuses them into a patient.
Some trials of this technology have shown dramatic success, including appearing to cure two infants who had leukemia earlier this year. And Novartis just received FDA approval last week for what might be thought of as first-generation CAR-T therapy, in which a patient's immune cells are removed, modified, and infused back into them.
Off-the-shelf CAR-T works a little differently, using T cells from donors instead of from the patient themselves. If it proves safe and effective, it would be far easier to administer than the personalized version of CAR-T, which is tailor-made for each patient.
Sadly, though, the death in the Cellectis trial is far from the first in the world of CAR-T.
Last year, trials of personalized CAR-T by the biotech firm Juno resulted in a patient death. Researchers made a quick change to the trial and the FDA allowed it to resume, but three more deaths soon followed. As Endpoints observes, that experience may mean that Cellectis will have a hard time restarting its trials until regulators are convinced the company has better prospects for avoiding a tragic outcome.