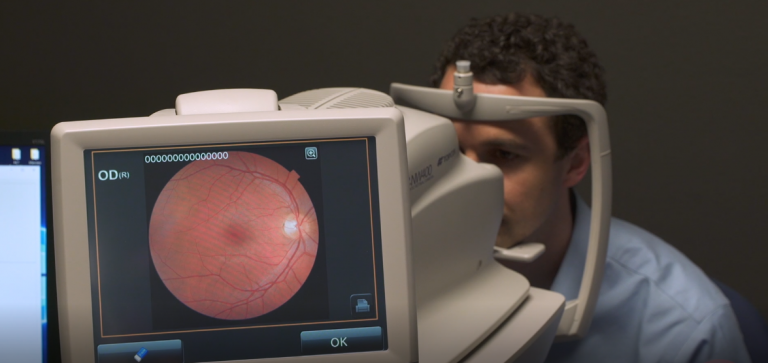
Marking a new era of “diagnosis by software,” the US Food and Drug Administration on Wednesday gave permission to a company called IDx to market an AI-powered diagnostic device for ophthalmology.
What it does: The software is designed to detect greater than a mild level of diabetic retinopathy, which causes vision loss and affects 30 million people in the US. It occurs when high blood sugar damages blood vessels in the retina.
How it works: The program uses an AI algorithm to analyze images of the adult eye taken with a special retinal camera. A doctor uploads the images to a cloud server, and the software then delivers a positive or negative result.
No specialist required: The FDA recently cleared AI-based software to help detect stroke, too. But the agency says this is the first device authorized to provide a screening decision without the need for a specialized doctor to interpret the image or results.
A look ahead: In a series of tweets today, FDA commissioner Scott Gottlieb hinted that more AI devices could get the agency’s seal of approval soon. Gottlieb said the FDA is “taking steps to promote innovation and support the use of artificial intelligence-based medical devices.” Not to worry, though: AI diagnostics probably won’t be replacing doctors or other medical professionals anytime soon.